Supreme Court hears arguments Tuesday in case that could restrict access to abortion medication
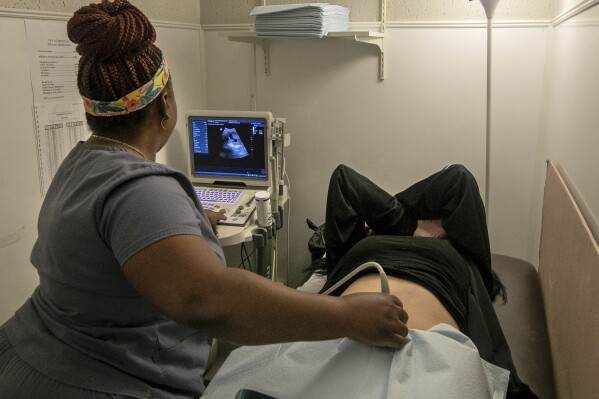
WASHINGTON (AP) — The Supreme Court is hearing arguments Tuesday in its first abortion case since conservative justices overturned the constitutional right to an abortion two years ago. At stake is the ease of access to a medication that was used in nearly two-thirds of all abortions in the U.S. last year.
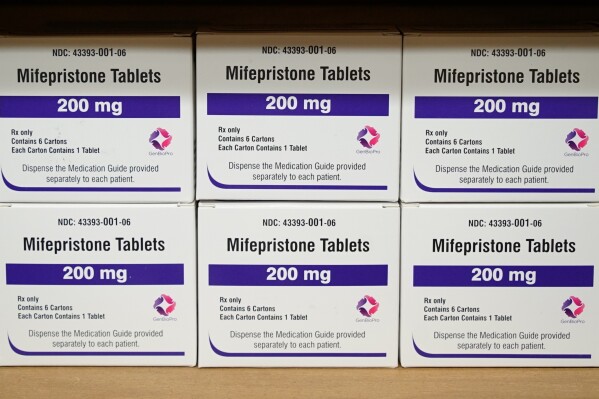
Abortion opponents are asking the justices to ratify a ruling from a conservative federal appeals court that would limit access to mifepristone, one of two drugs used in medication abortions.
The high court’s return to the abortion thicket is taking place in a political and regulatory landscape that was reshaped by the abortion decision in 2022 that led many Republican-led states to ban or severely restrict abortion.
That ruling had immediate political consequences and the outcome in the new case, expected by early summer, could affect races for Congress and the White House.
The practical consequences of a ruling for abortion opponents would be dramatic, possibly halting the delivery of mifepristone through the mail and at large pharmacy chains, reducing the period in pregnancy when it can be used from 10 to seven weeks and ending increasingly popular telehealth visits at which the drug can be prescribed.
The administration and drug manufacturers warn that such an outcome also could undermine the FDA’s drug approval process more widely by inviting judges to second-guess the agency’s scientific judgments.
Anti-abortion doctors and medical organizations argue that the FDA’s decisions in 2016 and 2021 to relax restrictions on getting the drug were unreasonable and “jeopardize women’s health across the nation.” The administration and New York-based Danco Laboratories, which makes mifepristone, respond that the drug is among the safest the FDA has ever approved.
In one possible resolution, the justices could avoid touching on the more politically sensitive aspects of the case while preserving access to mifepristone. The administration and Danco argue that the challengers lack the legal right, or standing, to sue. If the high court agrees, it would essentially dismiss the case and erase the appellate ruling.
Another abortion case already is on the docket. Next month, the justices will hear arguments over whether a federal law on emergency treatment at hospitals must include abortions, even in states that have otherwise banned them.
The mifepristone case began five months after the Supreme Court overturned Roe v. Wade. Abortion opponents initially won a sweeping ruling nearly a year ago from U.S. District Judge Matthew Kacsmaryk, a Trump nominee in Texas, which would have revoked the drug’s approval entirely. The 5th U.S. Circuit Court of Appeals left intact the FDA’s initial approval of mifepristone. But it would reverse changes regulators made in 2016 and 2021 that eased some conditions for administering the drug.
The Supreme Court put the appeals court’s modified ruling on hold, then agreed to hear the case, though Justices Samuel Alito, the author of the decision overturning Roe, and Clarence Thomas would have allowed some restrictions to take effect while the case proceeded.
Mifepristone is one of two drugs, along with misoprostol, used in medication abortions. Their numbers have been rising for years. More than 6 million people have used mifepristone since 2000. Mifepristone is taken first to dilate the cervix and block the hormone progesterone, which is needed to sustain a pregnancy. Misoprostol is taken 24 to 48 hours later, causing the uterus to contract and expel pregnancy tissue.
Health care providers have said that if mifepristone is no longer available or is too hard to obtain, they would switch to using only misoprostol, which is somewhat less effective in ending pregnancies.
Disclaimer: The copyright of this article belongs to the original author. Reposting this article is solely for the purpose of information dissemination and does not constitute any investment advice. If there is any infringement, please contact us immediately. We will make corrections or deletions as necessary. Thank you.


