Scientists Find Success With New Direct Ocean Carbon Capture Technology
As human activity and climate change increase the amount of carbon dioxide in the ocean, harming coral reefs and marine life, researchers have designed a new technology using aqueous sodium hydroxide and sodium carbonate to remove carbon dioxide from ocean water, helping reverse acidification and reduce global warming.
“It took years to pull this off experimentally,” said Katherine Hornbostel, assistant professor of mechanical engineering and materials science at the University of Pittsburgh’s Swanson School of Engineering. “So it was super rewarding to see when the experimental results finally matched our model[s].”
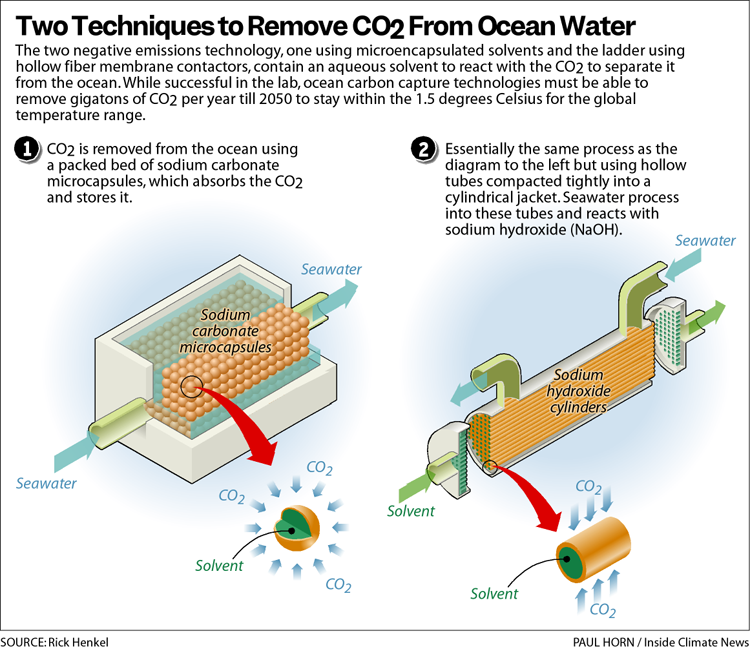
Hornbostel pursued developing the technology five years ago, when there was minimal research or funding for capturing carbon dioxide from the ocean, she said. There are two models, one using microencapsulated solvents made of sodium carbonate and another using hollow fiber membrane contactors containing sodium hydroxide. The two models are different from one another in geometry but function the same way, Hornbostel said.
For the first model Hornbostel, along with Assistant Professor Tagbo Niepa from Swanson’s Department of Chemical and Petroleum Engineering, developed capsules carrying a solvent made of the sodium solution. Niepa said he initially designed the capsules as an assessment tool for scanning the human body but was able to rework them as vehicles to decarbonize the ocean.
Hornbostel said the capsules are like extremely tiny caviar beads, containing the sodium carbonate liquid for the carbon dioxide to react with.
“So you want to pack a lot of these little beads” into the capillary tubes for the seawater to flow over so that there’s “a ton of points of contact,” Horbonstel said. “More CO2” will pass through and diffuse “from one to the other capsule because basically it wants to react with the liquid inside of the capsules.”
The sodium capsules can be regenerated by steaming it to 100 to 120 degrees Celsius which would allow the captured carbon dioxide to be removed and placed in storage, the paper says. Regeneration also allows for the capsules to be reused again to capture more carbon dioxide in future cycles.
The “hollow fiber membrane” allows seawater to enter and react with the sodium hydroxide solvents in the cylindrical capsules. The membrane allows a mass transfer of seawater to react with the solvent, Hornbostel said.
The hollow fiber membrane is made of a flexible polymer similar to polypropylene that allows gas to pass through it, but not liquids or ions. “We are the first team to our knowledge to demonstrate CO2 removal from seawater using a membrane contactor with a CO2 solvent,” the research team’s paper says.
Horbostel said having a high surface area for the seawater is significant, because it allows for diluted carbon dioxide to be removed from the water faster. “Mass transfer scales directly with surface area, so if you double the surface area of a membrane, it will double the rate that carbon dioxide crosses it,” she said.
Seawater “has high concentrations of bound CO2 … that can potentially be accessed” through direct ocean capture technology, the paper says. “Additionally, seawater is 1,000 times denser than air, which could result in a smaller system size.”
Direct ocean capture, the paper says, “could also potentially be performed offshore” on an abandoned oil platform, for example, to avoid using land space, and be co-located with offshore wind and/or offshore storage” of captured CO2 gas.
Ensuring the technology works at a larger scale in the ocean at an affordable cost is challenging but not impossible, Hornbostel said. She and the team will examine ways to increase the pH of ocean water, which enables the release of more carbon dioxide. Although still in the planning stages, the model can be placed offshore in a region recognized to have a high concentration of carbon dioxide in the ocean, Hornbostel said.
“As the largest part of Earth’s natural carbon cycle, the ocean has an enormous potential to contribute to carbon dioxide removal at the gigaton-scale required,” David Koweek, chief scientist at nonprofit OceanVisions and expert on Hornbostel’s work, said. “Yet much of this potential remains unrealized because of low levels of research and development into ocean-based carbon dioxide removal technologies.”
Hornbostel’s paper says that 10 gigatons of carbon dioxide must be removed from the environment each year by 2050 to ensure the global temperature does not rise more than 1.5 degrees Celsius, the most ambitious goal of the 2050 Paris climate agreement.
Studies show the ocean covers over 70 percent of Earth’s surface and has absorbed approximately 25 percent of carbon dioxide due to human activity. The ocean and atmosphere seek equilibrium by constantly exchanging carbon dioxide and various other gasses. Because carbon dioxide found in the atmosphere and ocean are linked, Hornbostel said carbon capture technology works in both the oceans and the atmosphere.
“Our technology can be thought of as ‘indirect air capture’ because the ocean will take more carbon dioxide out of the air above it to replace the carbon dioxide that we’re removing.” Hornbostel said.
Once carbon dioxide in the atmosphere reaches the ocean, it dissolves into a carbonic acid, lowering the pH level of the ocean to make it more acidic and therefore less habitable for marine life, according to the U.S Department of Commerce’s National Oceanic and Administration.
Peter Petraitis, professor of biology at the University of Pennsylvania, has seen a correlation between increased dissolved carbon dioxide in the ocean and a decline in mussels and gastropods in the Gulf of Maine over the last two decades. Petraitis and his team have continued to collect data since their study was published in 2020 and noticed gastropodes are declining at a far greater rate than hypothesized.
“Water temperatures are going up and [gastropods] are declining so the correlation is pretty strong,” Petraitis said.
Petraitis’s research found that the Gulf of Maine suffers from low pH and faster warming than 99.9 percent of all global oceans, and as time progresses, it will become less capable of mitigating the effects of ocean acidification.
The current pH level of the global ocean is at approximately 8.1, a drop of 0.1 since the start of the Industrial Revolution, according to the Environmental Protection Agency. The subtle change in pH level is alarming, Hornbostel said, as one pH unit decrease equates to ten times increase in acidity. The mild acid formed when carbon dioxide dissolves in water neutralizes calcium carbonate and bicarbonate, which corals and other invertebrates use to build their hard shells and skeletons.
Hornbostel’s team’s research found that its technology is promising but will require building capsules in large batches and investigating how the technology can be combined to work with existing infrastructure, like desalination facilities. Their paper calls for more research into seawater carbon dioxide capture technology, which is imperative given the rapid warming and acidification of the oceans.
Disclaimer: The copyright of this article belongs to the original author. Reposting this article is solely for the purpose of information dissemination and does not constitute any investment advice. If there is any infringement, please contact us immediately. We will make corrections or deletions as necessary. Thank you.







